Studies On Stability And Degragation Product Of Triamcinolone In Methanol
Abstract
Triamcinolone is a synthetic corticosteroid and a derivative of cortisol. It used to treat a number of human diseases, animal diseases and is also illegally added to personal care products. A methanol solution is normally used for analysis of triamcinolone.During stability study, we found that the triamcinolone solution in methanol was transformed to a new species with quite differentretention time on HPLC, which may lead to inaccurate qualification and quantitation. Herein, we report the study on stability of triamcinolone in methanol and acetonitrile, and the preparation and characterization of the degradation product.
Results and Discussion
1. HPLC Method for Triamcinolone Analysis
Triamcinolone in methanol solution is used for analysis either in GB/T 24800.2-2009 [1] or in Chinese Pharmacopoeia [2],no degradation phenomenon was described in both literatures. However,
during our analysis of standard solution of triamcinolone in methanol we found that there is a new peak appeared before the product with the same molecular weight. The HPLC method was optimized to ensure the complete separation of the two peaks (Fig. 1).The peak at 5.35 min belongs to triamcinolone, and that at 5.19 min belongs to the degradation product. The Mass spectra show that both peaks have the same molecular ion, [M + H]+= 395.2. This observation is coincident with the literature report that triamcinolone may occur cyclic rearrangement in suitable conditions [3].
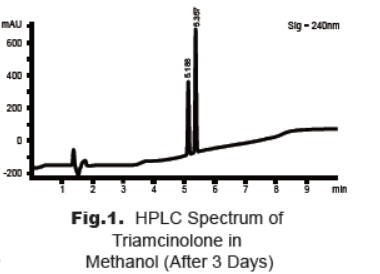
2. Stability Study of Triamcinolone Solution
To study the stability, a solution of triamcinolone in methanol or acetonitrile was prepared individually. The solutions were stored at 2-8°C and analyzed (Fig.2, Fig.3). The results clearly reveal that triamcinolone is not stable in methanol and it’s relatively stable in acetonitrile solution. Therefore, it’s suggested to use triamcinolone solution in acetonitrile rather than methanol for analysis and store the solution at -20oC or lower, or use freshly prepared solution.
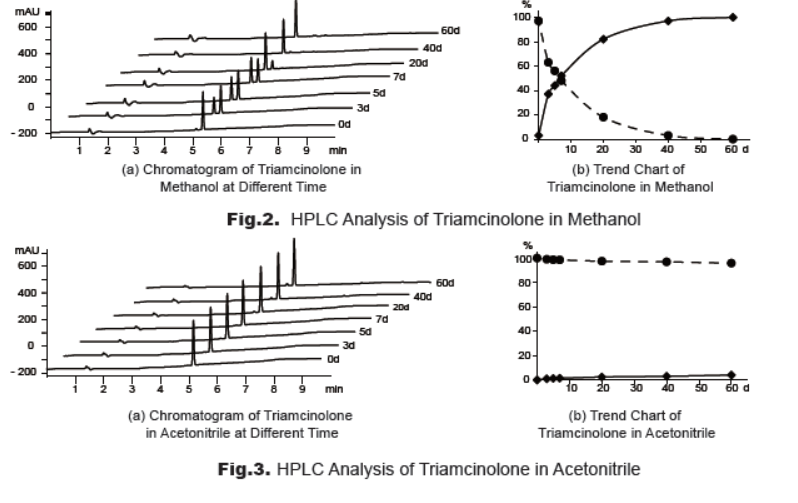
3. Synthesis and Characterization of Triamcinolone Analogue
To determine the degradation product, a dilute solution of triamcinolone A in methanol was stirred at room temperature for 3 weeks (Fig.4). After removal of solvent, the mixture was purified by prep-TLC. The new spot was collected to afford compound B as a white solid. 1HNMR spectra (Fig.5) demonstrate that the chemical shifts of the protons (C11-H, C16-H, C19-H2) next to hydroxyl groups change apparently. The multiplet of C11-H in structure A shifts from 4.95 ppm to 4.25 ppm. The triplete of C16-H in A shifts from 4.28 ppm to 4.05 ppm, and the two doublet of C19-H2 at 4.65 ppm and 4.29 ppm in A are shift to 3.90 ppm and 3.71 ppm, respectively.
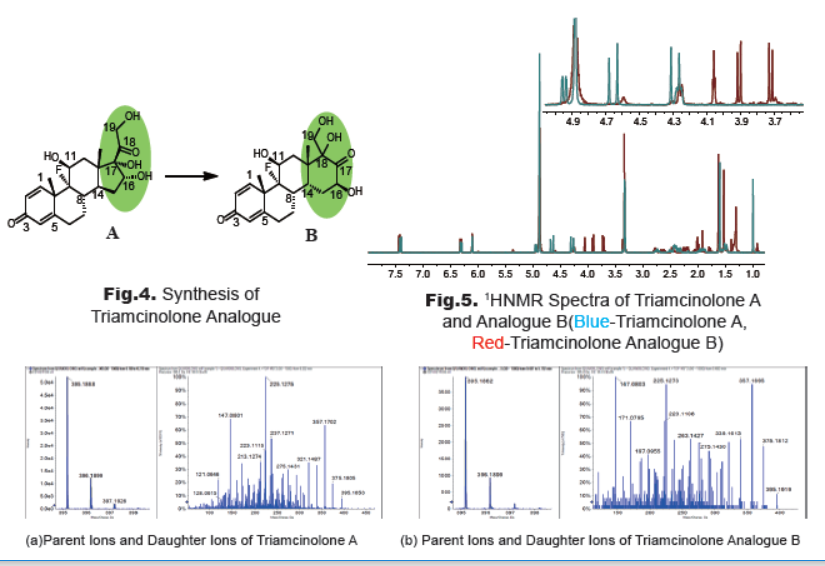
Fig.6. MS-MS Spectra of Triamcinolone A and Analogue B with Optimized Condition
The HPLC spectra demonstrate that compound B appears at the same position of the impurity. The HRMS spectra (Fig.6) show that compounds A and B have not only the same parent ions, but also the same fragmentation peaks of daughter ions. The preliminary results infer that the impurity maybe ring rearrangement product B, coincident with the literature report [3].
Conclusion
Triamcinolone is more stable in acetonitrile solution than in methanol. It transforms to a cyclic rearrangement product in methanol rapidly. Both triamcinolone and its degradation product
have the same parent ions and daughter ions. This should be considered in triamcinolone analysis for accurate qualification and quantitation. The degradation product was synthesized
and preliminary identified and characterized by HPLC, HRMS and 1HNMR to be a cyclic rearrangement analogue. Further experiments are on-going for full characterization.
References
1. GB/T 24800.2-2009, Determination of 41 glucocorticoids in cosmetics by LCMS-MS and TLC method.
2. Chinese Pharmacopoeia, 2010 Edition, page 267
3. Leland L. Smith, Michael Marx, etc. Journal of the American Chemical Society. 82: 4616-4625 (1960)




